Happy Thanksgiving, Statecraft readers!
I’ve been trying to get a conversation with today’s interviewee, Eric Van Gieson, PhD, since March. Van Gieson is a remarkable character, with a crazy CV: more than 25 years of experience in developing medical technology, and stints at multiple federal agencies including DARPA.
You may know that an American nonprofit, EcoHealth Alliance, received funding from the National Institutes of Health (NIH) for bat virus research, and that it was working on that research with collaborators in Wuhan, where COVID broke out. Before EcoHealth received NIH funding, it pitched a project to DARPA involving the insertion of human-specific cleavage sites into SARS-related bat coronaviruses. While Dr. Van Gieson was at DARPA, that proposal came across his desk. We talked about it today.
Van Gieson is also largely responsible for developing the Containerized BioContainment System (CBCS), a novel system used to move Ebola patients. We get into that in this conversation, but we cover a lot else; it’s a longer interview than most we’ve published. We tried to trim it down, but there’s so much good stuff here: word for word, it’s one of the richest interviews we’ve done. A lot of people have spilled a lot of ink discussing what went wrong during COVID, but I think what Van Gieson lays out here is close to a comprehensive account of the reasons we blew it, and how not to blow it in the future.
We discuss:
Why is the federal “pandemic preparedness” apparatus so sprawling?
Why haven’t we learned from COVID mistakes, or even run reviews on what went wrong?
How would you revamp the federal apparatus to be ready for the next pandemic?
We don’t test whether generic drugs can fight pathogens. Why not??
How did Van Gieson and colleagues ship a flying Ebola hospital in 6 weeks?
How can we make sure DARPA-developed biotech doesn’t end up in the hands of adversaries?
For a printable transcript of this interview, click here:
Eric, you have a military and medical background; you’ve spent time in several different federal agencies and departments focused on biodefense. You were at the Defense Threat Reduction Agency (DTRA) early in your career.
I've got a map here from one of my colleagues of all the federal agencies that work on disease or pandemic preparedness, and it's this really sprawling apparatus. What’s your view of how that whole system works?
I want to be very clear that I never served in the military: I was a civil servant in the military and served the military through the Intergovernmental Personnel Act while I was an employee at Johns Hopkins Applied Physics Laboratory.
DTRA is the research and development component of the US military's effort to combat weapons of mass destruction. My division focused on chemical and biological threats. It’s a very interesting organization because it sits between very early, nascent research and what we call acquisition:
In the military, when a product like a fighter jet like the F-35 is ready for deployment into the military force, it goes through an acquisition process: testing, validation of performance, and then there's acceptance and acquisition. Ultimately, the product goes into full-rate production and military service members use that product.
In DTRA, we were before acquisition. The role of DTRA was to find early development components, from places like DARPA or the NIH, and to help turn them into a product. If it's a drug, for example, we help get a product through preclinical testing and into a stage where it's ready for Phase I clinical trials.
In many cases, DTRA’s acquisition authority’s and related investments are unique. If you're trying to build a treatment for Ebola before there's a major Ebola pandemic, like we were in 2011, you have to use government funds, because there's no commercial pull. There's no reason for the commercial sector to build those products, but yet they are needed. We saw the early investments that DARPA made in companies like Moderna pay off in COVID, for example — even though the commercial sector and the government were not asking for nucleic-acid encoded vaccines and antibodies!
Why is the military investing in anti-Ebola drugs, as opposed to someone else in the federal government?
It goes decades back to the time when many countries had active weapons of mass destruction: biological weapons, chemical weapons, even nuclear development efforts. Even though there was a convention where parties signed and agreed to stop making biological threats, we know that chemical threats still exist — in the UK these weapons were even deployed to target specific individuals in 2018. The existence of these capabilities becomes a threat to our military service members who are doing their jobs every day. DTRA exists primarily to come up with innovative ways to protect those service members, through diagnosis, personal protective equipment (PPE), therapeutics, and vaccines. The enterprise is there to protect them from manmade and even emerging threats.
Across the federal government, all kinds of departments and working groups are oriented around the same problem of biological threat reduction. How much do these agencies talk to each other and connect?
There's a difference between talking and actually coordinating. There's quite a bit of talking and quite a bit of discussion about coordination.
Each of the agencies typically has a public-facing conference. There are active efforts to stimulate interaction between the different responsible components of our Chemical, Biological, Radiological, and Nuclear Defense (JPEO-CBRND) infrastructure within the DoD, outside of the DoD, the rest of Health and Human Services, and the rest of the government.
Heroic efforts to stimulate conversation.
So sometimes different health agencies are just talking, not actually coordinating. I won't ask you to call out agencies that don't pull their weight, but if you were a lawmaker, how would you figure out which parts of this apparatus are delivering and which parts are doing a lot of talking?
The easiest way is to do actual scenarios. When I was at DARPA and at DTRA, we would test the components of a very complex system and see what was redundant, what was working, and what wasn’t working. It would lead to much improvement in efficiency and execution.
If you're a lawmaker, every year, you have plenty of opportunities, like seasonal outbreaks, to test the response apparatus. From care to protective equipment, to therapeutics, diagnostics, and the vaccination process, there are metrics. How many people got infected? What was your mortality rate? What was your deployment rate? What was your acceptance rate for all the products that I just mentioned? And were there supply chain issues? All aspects can be graded.
A regular performance assessment would be an easy way to evaluate the baseline operating components of a response. And honestly, if a response authority to pandemics existed, you could have the authority activate to some low level, to ensure that it was able to assume command and control and activate the different components.
There are a lot of ways to run a semi-live fire scenario, and then you can provide a performance metric back to Congress or back to the agency leadership. They could see how things are working, and then you’d be able to go back and improve and evolve.
You have more of an opportunity here to do this in the pandemic scenario or the infectious disease scenario than we do in major natural disasters. Those are happening more often, but every time those happen, they're real and they're really bad. With influenza, you have a less severe version of a pandemic that happens every year or multiple times a year. And so you have a chance to score yourself and improve. We haven't done that. And that surprises me.
Say a little bit more about that. It seems obvious that after the COVID pandemic, the government would do a range of reviews.
No, we haven't. This morning, I was just on a call with a group of people who were part of Operation Warp Speed. Everyone was lamenting the fact that we haven’t learned. We actually don't know what is going on in terms of what went wrong, what went right, and what to do about it in the next response. And I worry.
I'll call out the CDC here because I think that they need to be called out. They built tests for COVID and actually declared to industry in January and early February of 2020 that they were developing a test, and there was no need for help from industry. And then they proceeded to deploy tests that didn't work. When they were inspected by people in the FDA, they were found to be deficient in their manufacturing processes at many levels.
If we were able to test, evaluate, and assess, we could have seen that problem well in advance. It's also a lesson we could learn from. But we actually didn't learn from that lesson in COVID, because when the CDC tried to build monkeypox assays, guess what? They initially didn’t work, again. They had problems. The problem keeps repeating itself.
The CDC is not a response organization. It shouldn’t be billed as one. For one, most of the people there do research or evaluate policy or are epidemiologists. They're not response entities.
Secondly, every time a new pandemic or outbreak occurs, the CDC brings up the response through the part of the organization that is focused on that particular pathogen. If it's a coronavirus, the coronavirus group responds. If it's a pox virus, then they're going to go and use that part of the CDC. The idea of having a centralized response component that coordinates and provides consistency is not part of their DNA. I think this is why you see the same mistakes happening multiple times. It shouldn't be allowed.
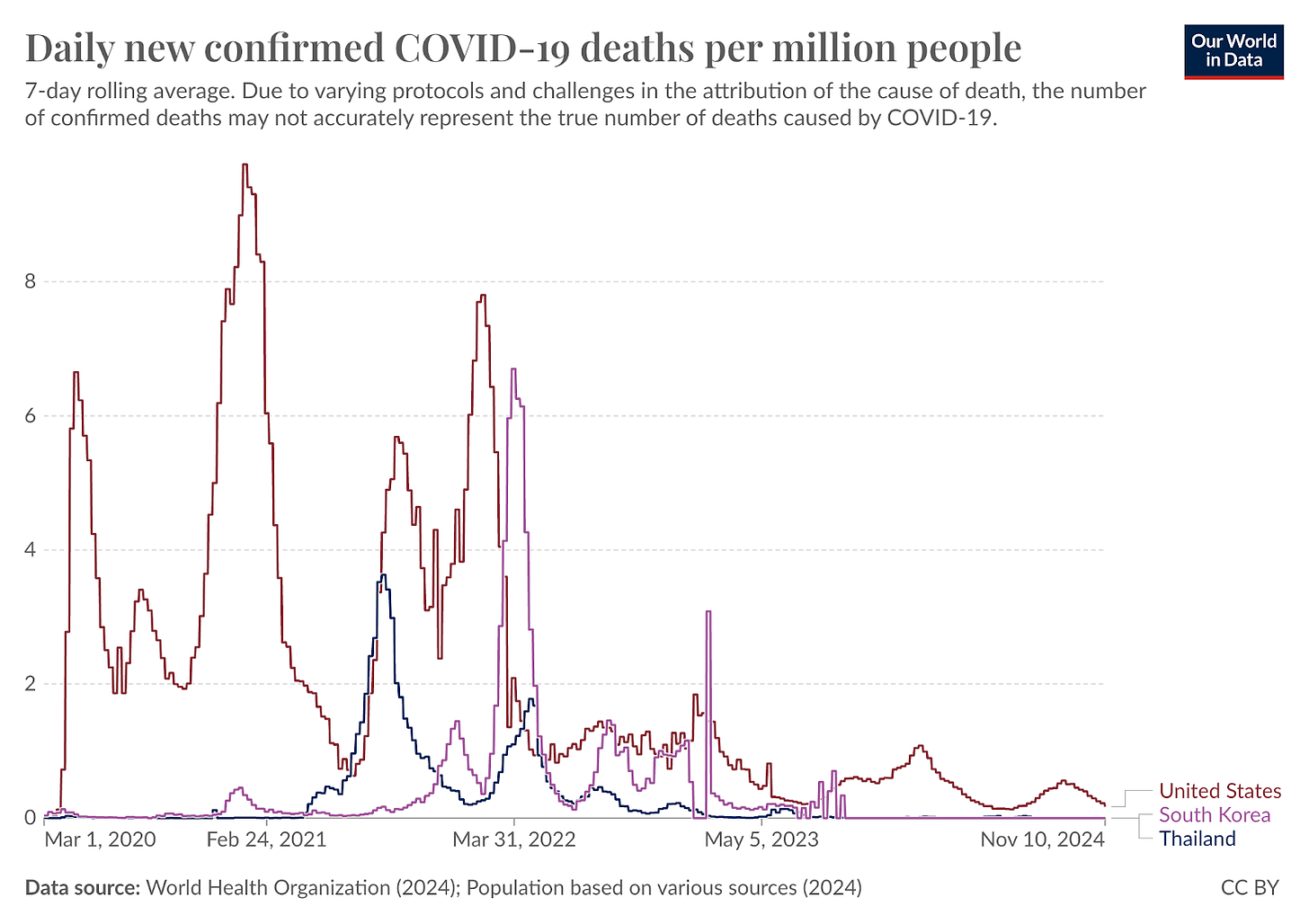
I want to make it very clear: if we could test the performance of our apparatus for response, we could catch these problems in times when it mattered less. It still matters, but it wouldn't lead to literally millions of lives lost. I'm not going to make the connection here overtly, but I will point out the countries that did deploy tests early, i.e. South Korea, Thailand, and Taiwan. [Korea, Thailand, and Taiwan all saw fewer deaths per capita during COVID.]
From Our World in Data.
If you look at their mortality rates per capita, they were orders of magnitude less than ours in the first two years of COVID. We deployed testing later: the CDC said they had it, so industry didn't deploy. If your country waited until March to deploy testing, you lost hundreds of thousands more people compared to the countries that successfully deployed working tests.
We continue to talk about the priority of deploying vaccines early, when we should be prioritizing deploying tests early. You will not have a vaccine unless you’re facing an existing pathogen that you already know the vaccine works against. If it's an unforeseen emerging threat, the first thing you'll be able to do is test for it, and then you'll be able to do therapeutics, and then you'll be able to do vaccines. That is just the way things have worked.
If we exercise that, we could identify the weaknesses in the system and fix them before they erupt. We'd also be able to bring in industry. We built close relationships with industry during things like COVID-19, but those relationships fade over time: New people get hired in industry; new people come into government. To maintain that public-private relationship, doing a demonstration or a live fire test with industry would be critical to maintaining those relationships.
Other countries do that, like Korea. They have long-term contracts. They call them IDIQ — Indefinite Deliverable, Indefinite Quantity contracts — which allow for the activation of an industry partner or commercial partner when needed. It can go dormant when it's not needed. You could activate that kind of relationship for testing. It would all be there when we need a response. The relationship would be warm.
Why does CDC not learn from these institutional mistakes? And why do institutions like CDC say, “We'll do it ourselves. No, thank you, industry professionals. We've got it in hand.” Where does that institutional instinct come from?
I don't want to analyze the CDC because I was never part of it, and I don't want to troubleshoot them — but I will say what I know works in terms of response, which has been a DoD-oriented approach.
I've been inside DoD’s operational teams and seen their command and control structure in action. People rotate through the organization. There's no long-term territoriality, so it gives experts chances to move on and become experts in other things. A new expert appears, but that new person must still learn from their predecessor — there's a training process in place.
There’s what the military calls doctrine. Doctrine is there so that you have a general framework that you follow to respond to things, and it’s adapted to the unique situation by the individual who's implementing that doctrine. Because you have doctrine, testing evaluation processes, and a very specific training process, everybody in DoD knows their job. They’re ready for the next thing because they can fall back on the training and doctrine. The command and control structure is already established. Other organizations just don't have that.
That’s why I endorse this idea of a national response authority. If you're a research organization like CDC or NIH, you don’t need doctrine. You want people to be able to think more openly about what they want to do in their research, and what problems they want to try to solve. At DARPA you have a general framework, but you're judged by your ability to create and to solve problems. You're not judged by your ability to respond to a threat — we had to in COVID, but that was just by accident.
You'd like to see this kind of national response authority, the live-fire testing. If you could wave a wand on American pandemic preparedness, what other reforms would you like to see carried out?
Better connection to industry. I mentioned what Korea is doing — IDIQ contracts with the commercial sector that enabled them to deploy diagnostics at scale way beyond anything we could do at the beginning of the pandemic: We did catch up to them, but it took us months. They were at scale in February — millions of tests per day were available in Korea in the early days of the pandemic. We weren't at that stage until April, maybe May.
Those two months: that's the invisible graveyard your friend talks about. It's unbelievable what an impact that had. And yes, there are ongoing efforts to build contractual engagements with industry, but they're not issuing IDIQs. I can tell you that for a fact. None of the agencies are doing that.
Why not?
I don't have an answer. I wish they were issuing IDIQs, because they would solve so many issues. At the beginning of the pandemic, we had to go into contract negotiations or look for existing contract vehicles in a given agency within the government to mod the contract and push as much money through as we could. It took weeks. In some cases, I think with PPE, the contracts took 10 weeks to get in place.
Luckily, people in the DoD helped people in HHS, but it shouldn't have to be that way. We should have industry ready to tap into. It should include all the major companies. If we have a large manufacturer of therapeutics, vaccines, or PPE, put them on contract. And you may never activate it, so it doesn't cost anything.
Take a step back for me: how do IDIQs work?
The government will put out a broad agency announcement, a request for companies to come back with a proposal. It can start with a request for information, and then it can lead to a broad agency announcement, and those announcements go to the world. The government would word this response like, “We're looking for companies that can make a million vaccine doses or lateral flow tests or PCR tests or sampling kits or masks per day.” Anyone who feels qualified to answer the request can respond.
You're looking for companies that can give you the production scale you need, and you judge them through their responses. You might give them $50,000 to start to help them develop a response plan, but it's not a significant amount of money. By giving them that small amount of money, it activates what we call the Indefinite Deliverable Indefinite Quantity contract — the IDIQ.
That IDIQ can have a high ceiling, say $10 billion. But whatever the number is, you're not obligated to hit that ceiling. It just allows you to add tasks as needed to the contract. All you're doing is issuing a tasker and saying, “We need a million COVID tests by X date.” And every company you have on contract already through the IDIQ has a chance to respond.
If they say they can do it, you can decide which of those companies to use and issue the task to that company, and they will do the job. Not only do they know that they're going to get paid, but you know you’re going to get the product from them, because you've pre-evaluated them for their capability of delivering that product, barring any sort of catastrophe. And if you ever need to do an exercise with them, you can use the IDIQ you already have to do the exercise.
It's just strange to me, because we go to the same companies every time we have a major disease event. We always turn to the same players and say, “How can we get you on contract? We need you right now.” But we could have had them on contract 10 years ago. It's like Groundhog Day.
That would be the magic wand I’d wave, but there's more: We should be screening every generic therapeutic on the market against all known pathogens. We could do a lot of that work in silico using supercomputing capacity at Lawrence Livermore and do in vitro testing in places like Texas Biomed and other parts of the country that have high containment facilities. We could have a shortlist of drugs that might work against different pathogens.
You could do clinical safety studies. For example, when monkeypox was mostly isolated to Africa, we could have been testing therapeutics at a significant scale for safety and efficacy to try to help those individuals, while simultaneously preventing potential spread. We're not doing those things. They're easy to do, and we're not doing them.
We don't test on-market generics for what else they’d work against? We don't just store that information somewhere?
We don't have a systematic process to do that. It’s a huge opportunity — every pandemic we've had since swine flu, since like '08, '09 — for every pandemic or outbreak we've had, we’ve found a generic therapeutic that has worked, after the fact.
There's plenty of data supporting this concept that generic or branded on-market things are there and could have worked. But because we didn't do the diligence to work through every known family of virus and every known family or species of bacteria, we had to go scour the literature and hope somebody had done it. It wasn't systematically done so that we could just knock off threat by threat, drug by drug.
There are 10-20,000 generics out there. Chances are, they'll probably have some effects, and we could certainly explore them systematically through research, and we don’t.
That seems relatively low lift. It's not any new technical work. It's just pattern matching of existing tools to existing diseases.
Yeah. We have the in silico tools at Lawrence Livermore GUIDE (Generative Unconstrained Intelligent Drug Engineering), and there's another product called ATOM developed by John Baldoni out of GlaxoSmithKline. You’ve seen all of these AI-based drug modeling companies emerge from places like Stanford, but they emerged after GUIDE and ATOM.
During COVID, when Omicron appeared in 2021, the folks at Livermore decided over two days to redesign one of the commercial antibodies. It's maintained performance across many different variants. It's a powerful tool, and thankfully people in the DoD are starting to fund this concept, but they're really limited to weapons of mass destruction and biological threat agents, not the whole panacea of pathogens that are out there. Someone needs to go through the whole list. And run that against drugs and then start testing in vitro and in vivo.
Let’s go to one of these outbreaks that you had personal experience with, the Ebola crisis. Tell me about your involvement.
So when Ebola initially began, I was just leaving DTRA, where I did a three-year tour on loan from the Johns Hopkins Applied Physics Laboratory. After I finished, I decided to take a job with MRI Global, a company I've always admired for its ability to respond to threats and engineer innovative solutions.
The job started in 2014, and that was when Ebola was emerging in Guinea and then eventually in Sierra Leone and Liberia. By February or March of 2014, I sat down with Will Walters and Wes Carter, and we decided to think about what we could do. Wes had just come to MRIGlobal from the US Army Research Institute for Infectious Disease and was coordinating already with Will, who was at the State Department.
Will had already coordinated the evacuation of the first two US volunteers from West Africa back to Nebraska, but he had to use a small jet operated by Phoenix Air Group. This small Gulfstream jet couldn't quite make it across the Atlantic and had to stop in the Azores and refuel before landing in West Africa. It was a corporate jet modified with containment systems, a lot of plastic, and a lot of filtration to be able to house Ebola patients.
The CDC actually owned this resource but decided not to use it. So Will said, “I'll do it.” He took the mission on and he got those people out of there. He took over the contract with Phoenix Air Group and used the State Department authority to do it, which was a heroic act.
Now, Will was a US Army Special Forces doc, and he had created a group called the Directorate for Operational Medicine within the State Department. He brought in people from the military and ran it as a small military-like unit with people he had known from his command. It was a very efficient group.
Will realized in that first evacuation that if we had to move a lot of people, there was no way we could move them back to the US. We had to make this move for medical volunteers, because treatment capabilities in West Africa were limited and would strain existing resources focused on the local population. A lot of people weren't trained on how to handle infectious disease cases, so if people are volunteering in West Africa, how are they going to get back en masse if everyone gets sick?
He came to Wes, and Wes came to me, and we literally drew out the design of the containerized biocontainment system on a piece of paper — on a napkin, actually — Will has the napkin. We transferred that to a whiteboard, and then we brought in an engineering team to convert that into a CAD drawing. We brought in James Lawler, MD, MPH too, who's now at the University of Nebraska to help oversee and design it, because James is one of the most experienced infectious disease doctors out there, and he’d served in the military with his colleague, David Brett-Major, MD, MPH. They’re probably the two most clinically experienced infectious disease docs in hemorrhagic fever in the past decade or two.
We came up with this design in a shipping container footprint. It looked just like a shipping container, but it was actually built from scratch: an intensive care unit with filtration that met the guidelines of a biosafety Level 4 laboratory. It had all the things you needed to function on a 17-hour flight from West Africa to the US.
So you could treat a highly contagious, very sick patient in the air?
Yes. It had to go through airworthiness certification and had to deal with explosive decompression if it lost pressure in the air. It had to fit in multiple aircraft, like the commercial 747-8, but it also had to fit in military aircraft. What's remarkable about this whole effort is that it was built in six weeks — we went through all of those requirements and demonstrated them. And even more remarkable was that the US government didn't pay for it.
The initial two prototypes that were used were paid for by the Paul Allen Foundation. The money was given to the State Department, and then the State Department contracted my company to do it. We used a public-private partnership. I don't think it would ever have been built in that fashion at that speed, any other way.
Having a military leader overseeing this helped move things along, as he had his whole team with him, and they knew every move to make this work. In parallel, at MRIGlobal, we were also building laboratories that fit in those Conex boxes that could sit next to the Ebola treatment units in West Africa. We found that when people who thought they had Ebola went to these treatment units, they were going with symptoms that were very much like malaria.
A large fraction of those people were walking up to those units with malaria and then going into the unit because they didn't have a diagnostic system at the unit. They would give blood and get a diagnostic result — in some cases, 14, 15 hours later — because they had to take it all the way back to the lab on a motorcycle through wet, muddy trails — not really roads. And eventually, they'd get a result if it made it back.
So these people would go in with malaria, and some of them would come out or stay in with Ebola, and die. The mortality rate was much higher in West Africa than anywhere else. So if we could prevent people with only malaria from going into the treatment units to get exposed to Ebola, that was a win.
I argued very strongly, and Ian Watson listened. He was at DTRA at that time [2014]. He supported us in building diagnostic laboratories next to every Ebola treatment unit in West Africa. He was fully on board, and it was wonderful to see him take a risk there.
I am convinced that we saved many lives. We never quantified how many lives, but we know we diagnosed many people with malaria, and they didn’t have to get exposed to Ebola. It underscores the importance of having diagnostics available in a pandemic and not just resorting immediately to treatment. You have to have both.
We were doing both of those things: We were trying to figure out ways to diagnose people while they were going in for treatment, but we also were trying to figure out ways to move people who needed higher levels of care back to the US or back to Europe.
You built these biocontainment systems in a six-week sprint. That's an incredible turnaround. What did that look like?
It was remarkable and it started with the napkin. Within two days we were welding. Everybody was welding because even though I said they were shipping containers and Conex boxes, they weren't — they were bespoke systems that had to be built with stainless steel. They were double-walled, the welds were perfect, and all the corners were rounded so you could sterilize them. And it was able to withstand enormous amounts of pressure. Then you had to build battery backup systems because it had lithium batteries, which were still a little sketchy — we had to put lithium batteries on the system because if you lose power, you still need to run the blowers to keep the HEPA filtration system going to keep the people who are inside the system isolated from the crew or any other people on the aircraft.
And then there were all the medical components we had to literally go and buy: All of the oxygen systems, the oxygen lines. And then there was only one company that made aircraft-worthy litters, or beds for patients.
The individual who ran the litter company knew that he was the only game in town, so there was a lot of negotiating to get him to hurry up and build enough litters for us because he didn't have any — there were none available.
At the end of the day, that system was absolutely not easy to build — and then we had to epoxy coat everything, so that we could decontaminate it. We had to then test and verify our ability to decontaminate it.
It was accelerated development validation and production like I've never seen in my life. These people were heroes, every one of them.
The platform ended up getting an R&D 100 Award. Now it has gone through acquisition under the Air Mobility Command. In any case, there are many copies of this system throughout the military now, and it's a national resource. They used that system to carry people back from the Diamond Princess evacuation in the early days of COVID-19, so it was very useful in follow-on activities.
After 2014 when Ebola died down, I went back to Will Walters and said, “You're military and you understand we're going to have to exercise the system, right?” So, he developed a series of exercises.
He called them the Tranquil series. You had Tranquil Surge, Tranquil Dawn, and several other Tranquil exercises where he crawled, walked, and ran with the system. In one case, he just did a movement of mock patients between two US cities. He went from that to moving patients from West Africa back to containment centers in Nebraska or Atlanta.
And then he decided to do a large movement: he sent two 747s and several smaller aircraft to bring patients back to the US and distribute them to different isolation centers. He did all this between 2017 and 2019. He had a whole practice, a whole demonstration, and a testing team that was highly trained and effective so that when the flag went up for COVID, they were ready. It all worked out like it should have. That was the beauty of it. That part of the development process is something I’ve never experienced with such a high level of execution.
We've talked to other folks in this series who have done health work in Africa for the US federal government. They think that PEPFAR and our work on Ebola are signal diplomatic accomplishments. What's your read on that?
I think PEPFAR is the reason that we have so much credibility in Africa and the rest of the world, for that matter, in terms of controlling a major disease threat. It's an amazing capability that we have demonstrated time and time again, and all these follow-on efforts have occurred as a result.
Colonel Nelson Michaels and Colonel Julie Ake are implementing components of PEPFAR on the DoD side even now. Dave helped bring more of a military mindset to that process, which has been excellent. They believe in exercises, they believe in readiness, and they believe in developing doctrine.
I would argue that kept Ebola from leaking into Lagos in Nigeria because the PEPFAR labs that Julie and Nelson had created were there to do testing and catch early cases. And local clinicians were educated from that process as well.
What practically do you learn by doing in the PEPFAR example that you can apply in the Ebola case?
They would train not only in diagnosis but also in care procedures and isolation procedures. You would think with HIV, a non-contagious disease, that there's no reason for isolation, and that's generally true. But you still have to be careful with handling needles. You also have to wear PPE. They took advantage of being there for HIV by also providing training for other infectious diseases and developing the capability among clinicians to be aware of these other diseases and how to handle them if they occur.
They're not only training and testing, they're also trying to test the performance of new drugs. They're a small effort focused on HIV, so they can't systematically work through all the pathogens. But they’re trying to translate emerging and even existing drugs and test their safety and efficacy in real clinical environments in Africa. They also have other sites around the world and in the US where they run these trials.
In a sense, they're like a CRO (Contract Research Organization). By getting clinicians, care providers, and laboratories involved in the trials process, it refines their expertise and their ability to deal with these diseases, but it also makes them more familiar with the therapeutic options that are out there.
When you were on the ground, how did our engagement in Africa stack up with Chinese and Russian engagement? Did you cross paths?
Yeah. There's an example of where we were waiting to go in to meet with an African delegation. I won't name the country, but our team was prepared to speak in French — we had translators. And we got half an hour with the delegation from their Ministry of Health. We walked out, and the Chinese delegation came in, but they went to the effort of not only knowing French, but also knowing a local language that none of us had ever heard, and they spoke in that language to their hosts. We were ushered out. The Chinese stayed for three hours.
They had developed a deeper relationship than we had, mainly because they were outspending us. Obviously, they built the African CDC and have made some major investments in Africa. Now, are we losing that diplomatic battle? It's hard to say, but I can tell you that they’re putting an enormous amount of funding into Africa to develop as much credibility as they can on the infectious disease and clinical front, furthering the agenda that President Xi has laid out.
What are the clinical focuses for China? When I think of our engagement as a layman, I think Ebola, I think HIV/AIDS. Is there a particular focus for the Chinese?
It's the same as ours. They want to demonstrate to the world that they're leading in preparedness clinical research, showing they can build countermeasures to these diseases that you mentioned and others. But they also want to show they can provide early warning and the ability to survey for emerging diseases. They want to demonstrate all these things because it elevates their perception as a technologically advanced, resilient society.
I want to get into the stuff you're doing now. You're working in private sector biotech now. What does the biotech industry in America look like today? Taking this 3000-foot view, what are its strengths? What are its weaknesses?
During my time at DARPA, I learned that a lot of the biotech and med tech that came out of DARPA didn't get picked up by the US. Instead, it got picked up by others, like the Chinese and other adversaries, in perfectly legal ways: An investor comes to an investigator who's been funded by the US Government, becomes a majority owner of the company, and then the company's IP is effectively owned by that investor. If they represent another country, that IP can go to that other country. So the intellectual property is lost. And that's a big problem for me.
When I left DARPA, I refused to work for a standard biotech or med-tech company in that role. I focused on what I could do to help protect the intellectual property being developed here in the US. While I can't protect all of it, I can at least protect some of the things I worked on when I was at DARPA. Eventually, I have ambitions to build a fund focused on protecting intellectual property in the US by focusing on work that emerges from the US government. A lot of that work just sits there, available for anyone to take, and it's very good work.
In many cases, especially from agencies I worked in, I know the process of vetting who gets funded, testing the things that are funded, and making sure they go through "live fire testing." All those things mean the technology works. If it didn't work, it wouldn't have been funded early on, or it wouldn't have even been selected. So to me, there's a really rich space of technology there that is unused.
Initially, I'm trying to work on the technologies that I advocated for at DARPA. I didn't develop them — I paid people to develop them — but I want to make sure these technologies are successful in the real world. In my work in epigenetics, working with the founder of the DARPA biological technologies office on some other technology, we're trying to make sure those technologies get a commercial life, and then become available to the government when needed.
If it's good tech, commissioned by the federal government, and it's been vetted, why isn't this stuff getting picked up? Why does it get picked up overseas and not here?
Luckily, there are some heroes in the world, namely Ed You and Joe Hamel, who recognized this problem five to ten years ago. People in the Senate and the House have also stepped up (Sen. Marco Rubio is one example) where they've identified this problem and created legislation to protect against this, but it still happens.
It happens because we have a lot of people in government who are good at running government programs and picking and developing technology, but they've never been in the private sector. Or if they have, they haven't been in the parts of the private sector involved with technology development and transition: venture capital, private equity, startups. They don't know how that works.
Only now at DARPA, and at BARDA (Center for the Biomedical Advanced Research and Development Authority), where Joe started, along with Bob Kadlec, they started BARDA Ventures. Sandeep Patel [Editor’s note: we interviewed Sandeep] carried that through to really great success to show that they could actually create a public-private system that would take technology developed in the government and then commercialize it. DARPA is trying to do the same thing with a commercialization effort they have going on.
That's fairly nascent, and then you see the NSF (National Science Foundation) stepping up to do this. ARPA-H (Advanced Research Projects Agency for Health) is doing this with PATIO (Project Accelerator Transition Innovation Office). There are groups in the government that recognize the problem and are trying to solve it. It's just going to take time because this is a new concept.
Usually, on the government side, you're taught not to favor industry in terms of individual industry components: you need to be uniform in how you treat everyone in industry. But this is a personality shift in the government because all of a sudden you're saying, “No, we need to protect that technology. We need to protect that one and that one.” How do you do that without showing favoritism? You have to work through all these existential contractual challenges that go against all the training that everyone's had in government. It's a tricky process to develop.
But people are trying. I forgot to mention the Defense Innovation Unit. That's another really important component of the government that works with venture capital but also acts like a VC. And of course, In-Q-Tel is out there — it's not like no one's done anything, but it's hard to catch everything that's coming out of government R&D spending and protect it all.
NIH is probably the biggest R&D spender, at $50+ billion a year. And they don't have a robust infrastructure for formulating or tracking every piece of technology they develop and making sure that it stays in the US. It's not something they do. And, honestly, they fund work in many other countries as well. So that's something to consider in terms of how we’re protecting the IP that we're paying for as a government.
What are the tradeoffs between investing aggressively in cutting-edge biotech in the national interest and the risk of making some very bad investments, like we saw in Wuhan?
Oh yeah. Wuhan. That's a third rail in government right now — there's a big divide. And I won't go too deep into it, but ultimately, you have the government trying to invest in the most cutting-edge things possible, especially in places like DARPA and ARPA-H. And ironically, other parts of government that are supposedly R&D effort organizations are often very risk-averse in terms of the cutting edge.
In one sense, being cutting-edge doesn't equate to being dangerous, so you can develop new technology that is not dangerous at all. Especially if it's diagnostic, or if you're talking about certain medical devices, and even drugs, these are all very cutting-edge things. A lot of discovery has to happen, and many times, there's nothing dangerous about discovery.
It's when you’re working on things that could be considered dual-use technologies, or you're working on things that could alter naturally occurring organisms or systems that can make them dangerous. That's when the risk is enormous. Right now, across the interagency, there are different opinions about what is safe and what is not safe.
And this is public knowledge: the work that was done under EcoHealth Alliance — that work was going to EcoHealth Alliance, but the Wuhan Institute of Virology (WIV) was a subcontractor to EcoHealth Alliance. That work started in 2014 or 2015, and DARPA received a proposal from essentially the same consortium of performers. EcoHealth Alliance was the prime contractor, and WIV was one of the subcontractors. DARPA rejected that proposal.
So you saw that proposal?
Yeah. Now everyone's seen it, too, because it was FOIAed and published.
But while you were at DARPA, this came across the desk of a colleague.
Of many people, yes. Specifically, that proposal raised a lot of red flags. I can't speak about the DARPA proposal process, the feedback that was given, or any of the assessments, but if you read it and look at it through the FOIA process, you can see that there were many red flags in it. Speaking about it as a public citizen now, and not as a DARPA employee, I can tell you that there were a lot of concerns in that proposal.
People have denied that there was anything dangerous about the proposal — I don't think this debate will ever get resolved. But I think that it's an example of inconsistency in the context of safety, and the bottom line is that we should have consistency. That means that we need to reconcile the standards of what we consider safe across the interagency in the US. We need to think about how we’re building a safe research environment where we can be doing cutting-edge work. There should be a consistent approach to this across agencies.
If we look at the research done in the US by US researchers, there are so many guidelines and standards inside the US, and I can hardly think of any unsafe examples. And none of them have led to any sort of major unintended consequences.
If you're doing the work inside the US, it's generally going to be safe. We should probably be focusing our research dollars on building our own research infrastructure — both from a safety perspective and from a security perspective, we want to build up our biotech expertise, not someone else's.
At IFP, we often talk about ways to create more room for civil servants to do what they do best, but there’s a clear problem if they think what they do best is giving money to foreign subcontractors to do research. You have to crack down there.
You do. Even in 2014, we knew that China was rising as an adversary. And it's now quite obvious. And President Xi in 2021 declared a smokeless war on much of the world. This is not the kind of partner you want in research. You want a research partner who is aligned with your goals of curing disease and generally helping the whole world population.
It's a very careful balance between feasibility and innovation. And that is the line you always walk. Feasibility means, “Can you do it?” Feasibility also means, “Can you do it safely?” You're not just going out there and trying to build an invisibility cloak or a teleporter because that's not feasible — but you're also not trying to build a highly unstable fusion weapon because you know it's not safe. You're trying to build something that's going to innovate, but it's going to be done, and it's likely to succeed, but it's also likely to have very few unintended consequences, if any.
When you're building a drug for almost any disease, that's what you do. You have to balance safety and efficacy. If it's highly effective, you might tolerate a little bit of a safety risk, but not much. And the FDA is very good at walking that line, and so are the drug developers. That’s the key — it's not like this is a foreign concept. This is done every day in industry, and it's done every day in government. But a few people go too far into the unfeasible world, and it's pretty obvious when you see it. That's the thing that kind of baffles me: there’s a contingent of individuals in the government who maybe miss that.
I think the easiest way to solve it is to work in environments where there are guidelines. We know the guidelines in the US, Canada, and a lot of European partner countries — even in places like South Korea, Japan, and Singapore, where our Asian partners modeled their guidelines after ours. In some cases, we modeled our guidelines after theirs. If the guidelines for research are there, usually the safety component of feasibility is already in place.
What lessons from our response to COVID do you draw for productive collaboration in the future?
You saw a wonderful collaboration during the COVID response. That was driven by Dr. Bob Kadlec creating Operation Warp Speed because, to be honest, there wasn't an authority. And he's actually speaking right now at a UN meeting. The thesis of his conversation is that there needs to be a response and preparedness authority created that has the ability, upon the emergence of a threat or even in preparation for a threat, to act as a central point of coordination.
Having a central point of coordination and authority is similar to what FEMA has in disasters, but if you had a disaster that was related to a pandemic response authority, all of these different components of the apparatus would be able to work in an integrated fashion. Because you have so many different moving parts, you need to first mobilize diagnostics and PPE. If it's a public threat beyond a military threat, you need to mobilize education efforts to teach clinicians and the general public about how to deal with the emerging threat, and because the government doesn't have the capacity, you have to start with industry to scale up production of diagnostics, therapeutics, and vaccines.
In 2020, Dr. Kadlec created Operation Warp Speed ad hoc. Moncef Slaoui is the Oppenheimer of that effort. [NB: The Trump transition team reportedly asked Slaoui about his interest in the role of NIH director; Slaoui declined.] It led to a wonderful collaboration between the DoD and HHS. Many great individuals stepped up. In my case, I was working with Bruce Tromberg to try to help get his effort started with RadX as well as deploy our own diagnostic capabilities through DARPA. Using our contracting mechanisms, we redirected a lot of our funding to support efforts that ultimately benefited HHS. And we did that in diagnostics, therapeutics, and vaccine efforts. The JPEO and other parts of the military used their contracting vehicles in coordination with HHS to stimulate and initiate the production of other needed products, including PPE. This all was a beautiful symphony working together — ad hoc, but it worked.
I've heard Dr. Robert Kadlec speak about Operation Warp Speed. He has emphasized not only the cross-collaboration between all these different moving parts but also the role of the military in getting people’s rear in gear. It sounds like that's a piece of this too.
Well, for example, he brought in General Perna as part of Operation Warp Speed. When the military command structure and military individuals are brought in to solve a problem, there's no fear of the unknown. There’s only, “Hey, what do we need to do to solve this problem? We're going to treat it in an agnostic way. We're not going to pretend that there are any barriers to the solution. We’re just going to find a way to get it done.”
And that's exactly what happened. General Perna came in and brought that atmosphere of pretending this was a brand-new problem. “Let's think of all the possible ways to solve it. Everybody, pull together.” He would hold long interagency conference calls many times a day, just working through different issues.
It wasn't about who the leader was. If you had an idea, they wanted to hear it. And if it was possible, the idea was usually implemented. It led to getting antibodies out on the market in record time. Ironically, therapeutic antibodies actually beat mRNA vaccines to market. They were available in October of 2020.
I know mRNA is new and it’s exciting and it's configurable. But if you look at the numbers, a conventional biologic, an antibody [REGEN-COV-2, which President Trump famously received] was developed, and you could have made a vaccine — a conventional vaccine — in a very similar amount of time. mRNA was developed and approved and put into people through Emergency Use Authorization faster than the vaccine. I wouldn't say much faster. So that tells me that sometimes, more traditional tools are appropriate for a quick response. It also tells me that they were open-minded. The team at Warp Speed was willing to consider all options, not just the cool, fancy methodology that was mRNA.
But truth be told, the antibodies won. And by the way, it was an antibody from Regeneron, not an antibody from DARPA's funded efforts through Eli Lilly and Vanderbilt that actually won. Regeneron got the antibody out first, completely developed internally, not with any government funding, and they collaborated with BARDA (Biomedical Advanced Research and Development Authority) to get funding so that they could quickly get the antibodies to market. An industry-led effort got the product out there.
It just tells you that we need to consider all options. We need to make sure that the public-private partnership component is available. There's a lot of potential in untapped industry and we need to think about how to use that more efficiently.
I heard a story, possibly from Dr. Kadlec. Some essential input to mRNA vaccines was on a train headed somewhere, and the Army helicoptered in, invoked the Defense Production Act, and got it back into the mRNA production line. It sounds like that sort of thing doesn’t happen absent serious military involvement.
Absolutely. The Pfizer team was developing a shipment cooler, a cooler in which you could ship vaccines around the world. They brought it to Bob Kadlec and said, “Here's our shipment module. We're ready to go ahead and ship everything out.” And Bob said, “No, I want you to actually do one test shipment first. If all the bottles survive, ship everything in this configuration.”
Sure enough, they did the shipment. They brought it into Bob's office. They opened it: every single bottle was broken. That gave him a chance to adjust it to survive a shipment, but you can imagine vials of vaccine being broken all around the country.
Why hadn't they already done a test shipment?
In the military, you have research and development, then you have a very long testing phase, and you have what they call “live fire exercises,” where you're actually testing equipment. In the case of the military, bullets are flying, bombs are dropping, or the actual scenario of battle is exercised. You can do this concept in public health response, where you go through all the motions of a real scenario to make sure that every component works. Test to the last mile to make sure that everything works, leaving nothing to chance.
The military thinks like that. The rest of the world doesn’t always think like that.
Another saying that my friends in the military use often is, “One is none: always have redundancy.” Usually FEMA (the Federal Emergency Management Agency), they'll bring in two-star, three-star, and four-star generals to have significant roles in the response. You probably want to have a response authority similar to FEMA but for pandemics.
That mindset is built into these people. They just say, “I don't believe it until I see it actually demonstrated. I don't trust it.” If you're an academic or if you're in industry, you may have some element of that — most people in the military trust, but verify always.